What if Fanapt is the medication you've been looking for?
Trial and error is a challenge everyone with schizophrenia faces while working with his or her doctor to find the right treatment. Fanapt is a type of prescription medicine used for the treatment of schizophrenia in adults. Fanapt has been shown to treat the symptoms of schizophrenia.
Fanapt tablets are indicated for the treatment of schizophrenia in adults.
Deciding to look at alternate medications is something your doctor may do. He or she needs to consider that Fanapt may change your heart rhythm (meaning there is more time between heartbeats). When taking other drugs that may cause this same change in heart rhythm, you are at a higher risk of a serious, even life-threatening medical issue (torsade de pointes), which may result in sudden death. In many cases, your doctor may prescribe another medicine to treat schizophrenia before trying Fanapt.
Please see additional Important Safety Information below.
Fanapt needs to be taken as directed, starting at a low dose and slowly increasing the strength. This may delay the control of symptoms.
Starting Fanapt:
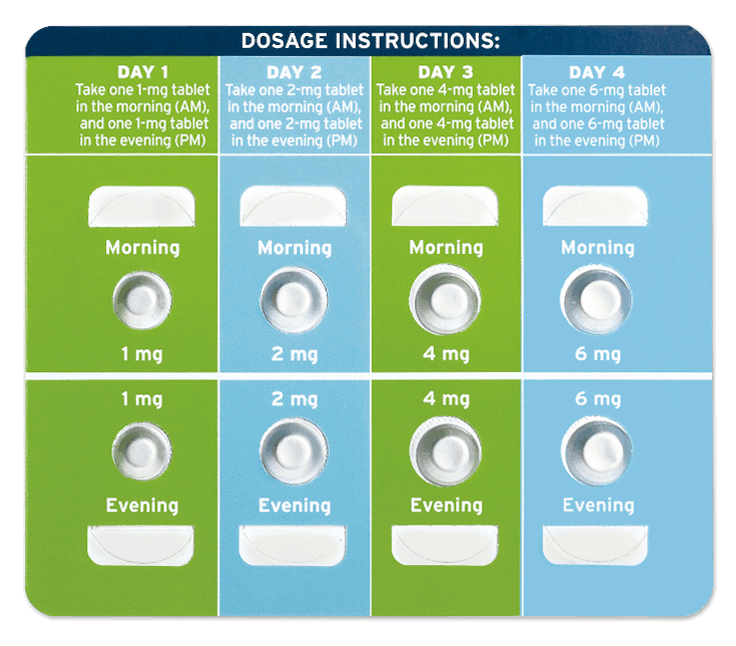
Fanapt is available in a specially designed starter pack. Treatment will begin at a lower dose of medication. You'll get used to Fanapt slowly by increasing the dosage strength over the course of 4 to 7 days, or as directed by your doctor. You must start at a low dose to help avoid lighteadedness or faintness caused by a change in heart rate and blood pressure when rising from a sitting or lying position.
Fanapt needs to be taken as directed, starting at a low dose and slowly increasing the dosage strength. This may delay the control of symptoms in the first 1 to 2 weeks of treatment.
How to take Fanapt
- Take 1 tablet in the morning and 1 tablet in the evening.
- Swallow the tablets whole, with or without food.
- Never change your dose or stop taking your meds without discussing it with your doctor.
- One of the most important things to remember is to keep taking your Fanapt even when you start to feel better.
Fanapt Starter Package:
Be sure to start taking Fanapt exactly as your doctor prescribes.
Tips to help make sure you don't miss a dose:
- Try linking in your mind the time when you take your meds to a daily activity, for instance after brushing your teeth in the morning and at night. Some people like to put their meds in a place where they'll always see them at that time, such as a shelf in the bathroom (out of children's reach).
- Set an alarm on a clock, watch, or phone to remind you when to take your meds. Or make arrangements with someone you trust to text you.
- Mark a calendar or journal when you take your meds.
- Use a pill holder to show whether you've taken your dose or not.
- Ask a family member or friend to remind you to take your meds.
- Use rubber bands to signal when you've taken your Fanapt. Start the day with two bands on your wrist, then take one off when you take each dose.
Fanapt and other medicines:
While you're on Fanapt, always check with your doctor before starting any new prescription or over-the-counter medicines you can get without a prescription, including any natural/herbal remedies. Some medicines may be unsafe to use when taking Fanapt. Some medicines can affect how well Fanapt works.
Overdose:
In case of overdose, call your doctor or poison control center immediately or go to the nearest emergency room.
Who should NOT take Fanapt?
- Elderly patients with psychosis related to dementia (having lost touch with reality due to memory loss and are experiencing a decline in day-to-day functioning).
- People with liver disease.
- Women who are pregnant, might be pregnant, or plan to get pregnant.
- People with a known allergy to Fanapt or its ingredients.
- Children and teenagers younger than 18 years.
Also, while taking Fanapt, avoid drinking alcohol and do not breast-feed.
RELAPSE PREVENTION
FANAPT® DEMONSTRATED LONG-TERM EFFICACY BY DELAYING TIME TO RELAPSE
FANAPT® DEMONSTRATED EFFICACY FOR LONG-TERM MAINTENANCE TREATMENT OF SCHIZOPHRENIA
KAPLAN MEIER ESTIMATION OF PERCENT RELAPSE/IMPENDING RELAPSE FOR FANAPT® AND PLACEBO-TREATED PATIENTS
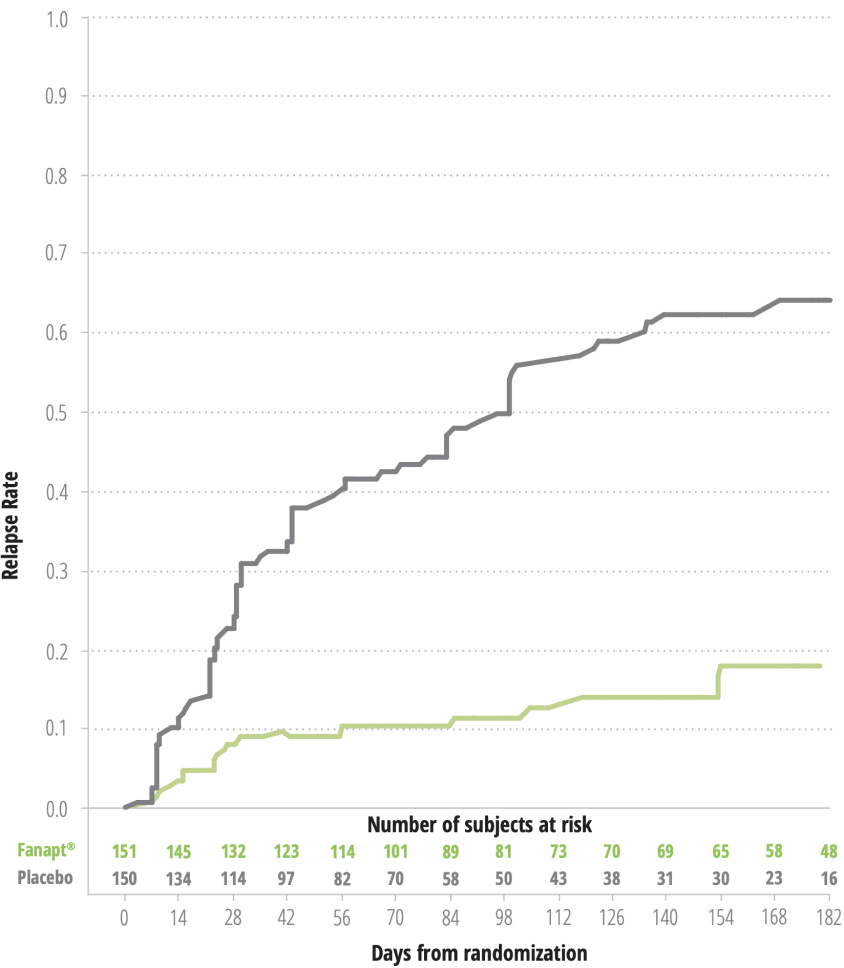
(log rank test: P<.0001)
*Based on final analysis dataset.
- Based on the interim analysis, The mean time to Relapse was 139 days for FANAPT® vs 71 days for PLACEBO4*
- After 6 months, 79.6% of patients taking FANAPT® did not Relapse vs 36.6% on PLACEBO, based on the interim analysis4
- No new safety signals compared to those observed in short-term stlidies4
- Flexible dosing of FANAPT® (8-24 mg/day) was effective1
*Relapse or impending relapse is defined as any of the following: hospitalization due to worsening of schizophrenia; increase (worsening) of the PANSS score ≥30%; CGI-improvement score of ≥6; patient had suicidal, homicidal, or aggressive behavior; or need for any other antipsychotic medication.
Including increase in study medication.CGI, Clinical Global Impression.
IMPORTANT SAFETY INFORMATION
Neuroleptic malignant syndrome, a potentially fatal symptom, has been reported in association with antipsychotic drugs, including Fanapt® . Manage with immediate discontinuation of drug, treatment if needed, and close monitoring.
Tardive dyskinesia: The risk of tardive dyskinesia may increase as the duration of treatment and total cumulative dose increases. Discontinue Fanapt® if clinically appropriate.
Seizures: Use Fanapt ® cautiously in patients with a history of seizures or with conditions that lower seizure threshold.
Falls: Fanapt ® may cause somnolence, postural hypotension, motor and sensory instability, which may lead to falls causing fractures or other injuries. For patients with diseases, conditions or medications that could exacerbate these effects, complete fall risk assessments initially and recurrently during therapy.
Leukopenia, neutropenia, and agranulocytosis have been reported with antipsychotics. Patients with a pre-existing low white blood cell count (WBC) or a history of leukopenia/neutropenia should have their complete blood count (CBC) monitored frequently during the first few months of therapy and should discontinue Fanapt at the first sign of a decline in WBC in the absence of other causative factors.
STUDY DESIGN: A double-blind, placebo-controlled, randomized withdrawl study (n=303) involving a flexible-dose range of Fanapt ® (8-24 mg/day) administered as twice-daily doses. The study consisted of a 7-day titration period where Fanapt ® was titrated at 1 mg twice daily on day 1,2 mg twice daily on day 2,4 mg twice daily on day 3,6 mg twice daily on day 4, 5, 6 and 7, followed by a stabilization period lasting at least 12 weeks. During the subsequent double-blind relapse prevention phase lasting up to 26 weeks, patients were randomized to placebo or established dose of Fanapt ® and observed for relapse or impending relapse. Stablization was defined as being on an established dose of Fanapt ® that was unchanged due to efficacy in the 4 weeks prior to randomization, having a CGI-Severity score ≤4 and PANSS score ≤70, a score of ≤4 on each of the following individual PANSS items (P1-delusions, P2-conceptual disorganization, P3-hallucinatory behavior, P6-suspiciousness/persecution, P7-hostility, or G8- uncooperativeness), and no hospitalization or increase in level of care to treat exacerbations. The primary endpoint was time to relapse or impending relapse compared to placebo, defined as any of the following: hospitalization due to worsening of schizophrenia; increase (worsening) of PANSS score of ≥30%; CGI-Improvement score ≥6; patient had suicidal, homicidal, or aggressive behavior; or need for any other antipsychotic medication, including increase in; study medication.
INDICATION AND IMPORTANT SAFETY INFORMATION
Fanapt® (iloperidone) is a prescription medication used for the treatment of schizophrenia and the acute treatment of manic or mixed episodes associated with bipolar I disorder in adults.
WARNING: Elderly patients with psychosis related to dementia (having lost touch with reality due to memory loss and experiencing a decline in day-to-day functioning) who are treated with antipsychotic medications are at an increased risk of death compared to patients treated with a placebo. Fanapt® is not approved for use in people with dementia-related psychosis.
WARNINGS AND PRECAUTIONS
- Patients should not use Fanapt® if they have a known allergy to Fanapt® or its ingredients. Allergic reactions, including anaphylaxis, rapid swelling of the skin (angioedema) and other symptoms of allergy (eg, throat tightness; swelling of the throat, face, lips, mouth and tongue; hives; rash and itching) have been reported.
- An increased risk of stroke has been reported in clinical studies of elderly people with dementia-related psychosis. Fanapt® is not approved for use in people with dementia-related psychosis.
- Fanapt® may change your heart rhythm (meaning there is more time between heartbeats). Heart rhythm changes have occurred in patients taking Fanapt® and are a risk factor for serious, even life-threatening medical issues. You should tell your healthcare provider if you have or have had heart problems. Contact your healthcare provider right away if you feel faint or have unpleasant feelings of irregular or forceful heartbeats as any of these feelings could be a sign of a rare, but serious side effect that could be fatal. You should not use Fanapt® with other drugs that are known to cause these same heart rhythm issues.
- Tell your healthcare provider if you have some or all of the following symptoms: very high fever, rigid muscles, shaking, confusion, sweating or increased heart rate and blood pressure. These may be signs of a condition called neuroleptic malignant syndrome (NMS), a rare but serious side effect that could be fatal. This may happen with Fanapt® or drugs like it.
- Abnormal or uncontrollable movements of the face, tongue or other parts of the body may be signs of a serious condition called tardive dyskinesia (TD), which could become permanent. The chance of this condition going away decreases, depending on how long and how much medication has been taken. Tell your healthcare provider if you have body movements you can't control.
- Fanapt® and medicines like it have been associated with metabolic changes (high blood sugar, high cholesterol and triglycerides, and weight gain) that can increase cardiovascular/cerebrovascular risks. Increases in blood sugar levels (hyperglycemia), which in some cases can be serious and associated with coma or death, have been reported in patients taking Fanapt® and medicines like it. Changes in cholesterol and triglycerides have been seen in patients taking Fanapt® and medicines like it. Some patients may gain weight while taking Fanapt®. Your healthcare provider should check your blood sugar, fat levels and weight before you start and regularly while you take Fanapt®.
- Tell your healthcare provider about any medical conditions that you have, including problems with your liver. Fanapt® is not recommended for patients with severe liver problems.
- Tell your healthcare provider if you have a history of or have a condition that may increase your risk for seizures before you begin taking Fanapt®.
- Light-headedness or faintness caused by a sudden change in heart rate and blood pressure when rising quickly from a sitting or lying position (orthostatic hypotension) has been reported with Fanapt®. This condition is most common when you start therapy, when restarting treatment or when the dose of Fanapt® is increased. You should consult your healthcare provider if you have or have had heart problems or conditions that lead to these sudden changes since Fanapt® should be used with caution in these patients.
- Fanapt® may increase the risk of falls, which could cause fractures or other injuries.
- Decreases in infection-fighting white blood cells (WBCs) have been reported in some patients taking antipsychotic agents. Patients with a preexisting history of low WBC count or who have experienced a low WBC count due to drug therapy should have their blood tested and monitored during the first few months of therapy. Some (including fatal) cases of agranulocytosis, a serious decrease in specific types of WBCs called neutrophils or granulocytes, have been reported in drugs like Fanapt®.
- Fanapt® can increase the level of the hormone prolactin. Tell your healthcare provider if you experience breast enlargement, breast pain or breast discharge, abnormal menstrual cycles in females or impotence in males. If elevated levels of prolactin persist, this may lead to bone loss.
- Medicines like Fanapt® can impact your body's ability to reduce your body temperature. You should avoid overheating and dehydration.
- Fanapt® and medicines like it have been associated with swallowing problems (dysphagia). If you have or have had swallowing problems, you should tell your healthcare provider.
- For males, in the rare event you have a painful or prolonged erection (priapism) lasting 4 or more hours, stop using Fanapt® and seek immediate medical attention.
- Fanapt® and medicines like it can affect your judgment, thinkingor motor skills. You should not drive or operate hazardous machinery, including automobiles, until you know how Fanapt® affects you.
- A condition called intraoperative floppy iris syndrome has been observed in some patients taking drugs like FANAPT®. Please tell your healthcare provider if you take or have taken Fanapt® before cataract or other eye surgeries.
- The most common side effects for Fanapt® versus placebo in patients with schizophrenia were dizziness, dry mouth, feeling unusually tired or sleepy, stuffy nose, feeling faint/light-headed when standing quickly, racing heartbeat and weight gain. The most common side effects for Fanapt® versus placebo in a clinical study of patients with bipolar I disorder were racing heartbeat, dizziness, dry mouth, increase in liver enzymes, stuffy nose, weight gain, low blood pressure and feeling unusually tired or sleepy. Compared to placebo, the average weight gain in clinical studies in schizophrenia lasting 4 to 6 weeks was 6 poundsand in a clinical study of patients with bipolar I disorder over 4 weeks was 7 pounds. If you experience any of these symptoms, talk with your healthcare provider.
- When taking Fanapt®, you should avoid drinking alcohol and you should not breastfeed.
- You should notify your healthcare provider if you become pregnant or intend to become pregnant while taking Fanapt®.
- Tell your healthcare provider about all prescription and non-prescription medicines and supplements you are taking. Some medications may interact with Fanapt®.
- To access the full Prescribing Information, including BOXED WARNING, visit www.Fanapt.com.
- You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.

